| 生物活性 | |||
|---|---|---|---|
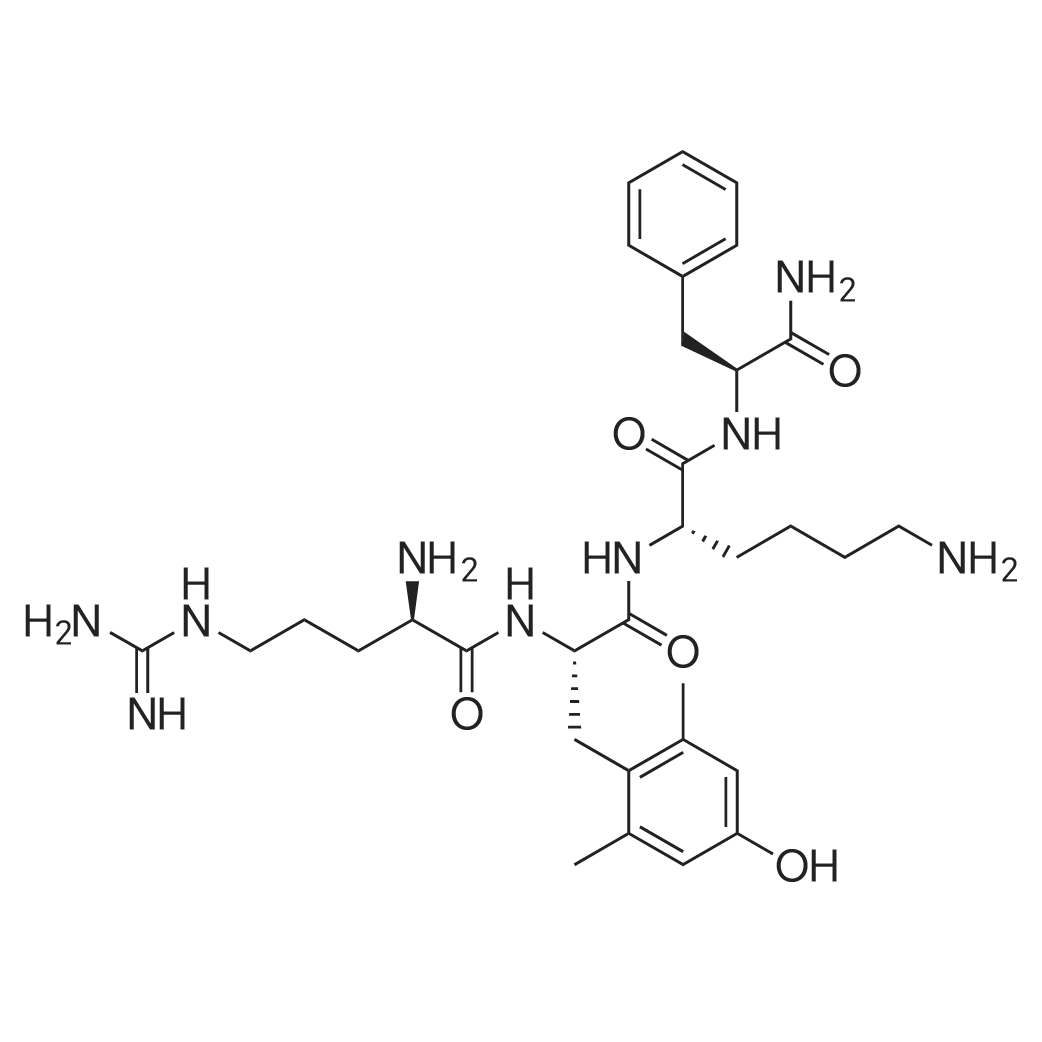
| 描述 | Impaired mitochondrial (MITO) energy metabolism plays a key pathogenic role in agerelated degenerative disorders and is centrally involved in organ ischemia/reperfusion injury. Abnormalities of MITO structure and function exist in the failing heart of humans and experimental animals evidenced by hyperplasia, reduced organelle size, diminished rate of ATP synthesis and increased formation of reactive oxygen species (ROS)[3]. Elamipretide (MTP-131), a novel mitochondria-targeting peptide, was shown to reduce infarct size in animals with myocardial infarction and improve renal function in pigs with acute and chronic kidney injury[3]. Long-term therapy with elamipretide improved left ventricular (LV) systolic function, normalized plasma biomarkers and reversed MITO abnormalities in LV myocardium of dogs with advanced heart failure (HF). The results supported the development of elamipretide for the treatment of HF[3]. Patients with heart failure with reduced ejection fraction (ejection fraction, ≤35%) are randomized to either a single 4-hour infusion of elamipretide (0.25 mg/kg/h) or placebo control. Compared with placebo, a significant decrease in left ventricular end-diastolic volume and end-systolic volume occured at end infusion in the highest dose cohort, which demonstrated that a single infusion of elamipretide was safe and well tolerated[4]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02367014 | Mitochondrial Myopathy | Phase 1 Phase 2 | Completed | - | United States, California ... 展开 >> University of California San Diego, California, United States United States, Massachusetts Massachusetts General Hospital Boston, Massachusetts, United States United States, Ohio Akron Children's Hospital Akron, Ohio, United States United States, Pennsylvania Children's Hospital of Pittsburg of UPMC Pittsburgh, Pennsylvania, United States 收起 << |
| NCT02245620 | Skeletal Muscle Mitochondrial ... 展开 >>Dysfunction in the Elderly 收起 << | Phase 2 | Completed | - | United States, Washington ... 展开 >> Seattle, Washington, United States 收起 << |
| NCT01755858 | Renal Artery Obstruction ... 展开 >> Hypertension, Renovascular Ischemia Reperfusion Injury 收起 << | Phase 1 Phase 2 | Terminated | - | United States, Minnesota ... 展开 >> Mayo Clinic Rochester, Minnesota, United States, 55905 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.56mL 0.31mL 0.16mL |
7.82mL 1.56mL 0.78mL |
15.63mL 3.13mL 1.56mL |
| 参考文献 |
|---|