| 生物活性 | |||
|---|---|---|---|
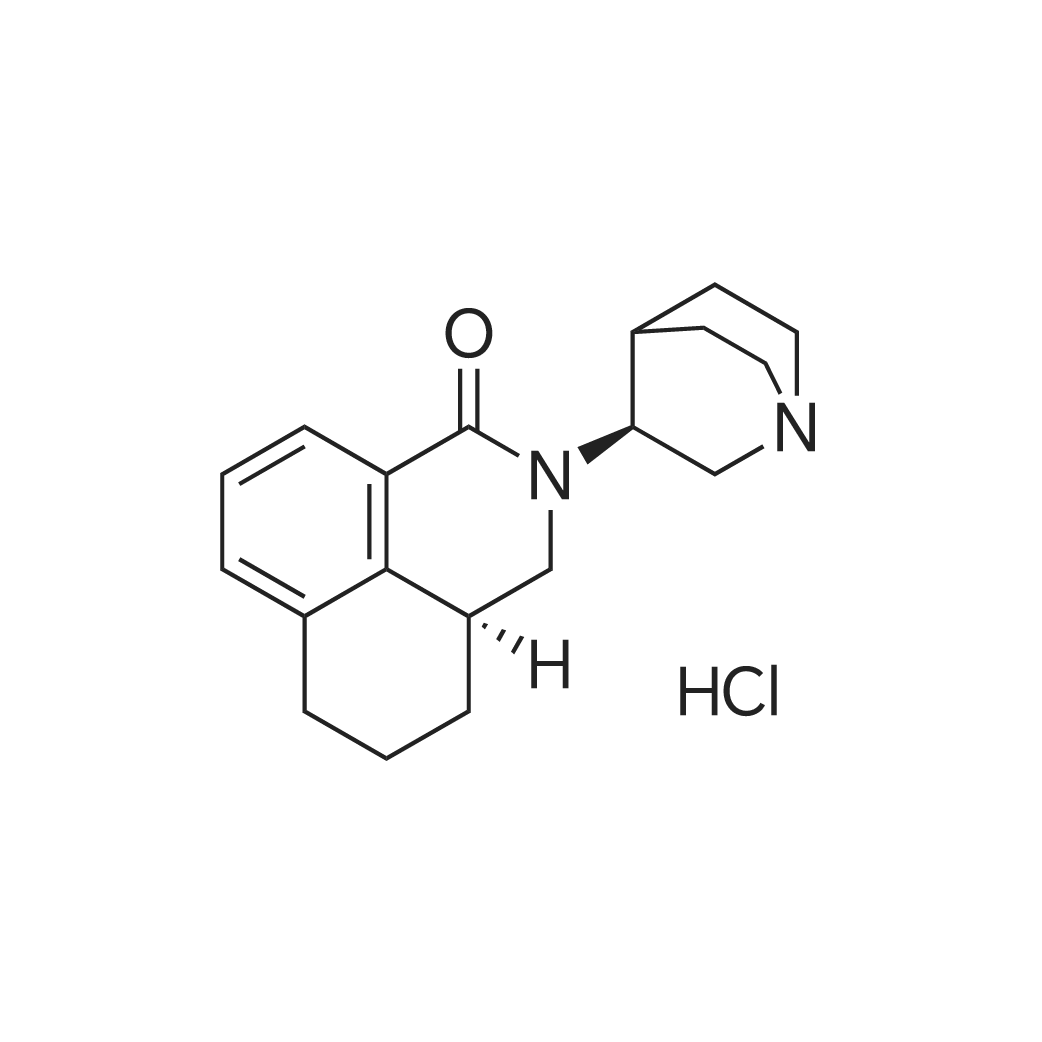
| 描述 | Palonosetron hydrochloride, as 5-HT3 receptor antagonist palonosetron, is an important treatment for emesis and nausea during cancer therapy. Chronic exposure to palonosetron reduced the number of available cell surface [3H]granisetron binding sites[3]. Combining low-dose palonosetron with droperidol potentiated prophylaxis for PONV (Postoperative nausea and vomiting) and achieved a similar prophylactic effect[4]. Moreover, palonosetron significantly adds to the clinician’s ability to effectively control CINV (chemotherapy-induced nausea and vomiting) in patients undergoing HEC or MEC (highly and moderately emetogenic chemotherapy)[5]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.00mL 0.60mL 0.30mL |
15.02mL 3.00mL 1.50mL |
30.04mL 6.01mL 3.00mL |
| 参考文献 |
|---|