| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
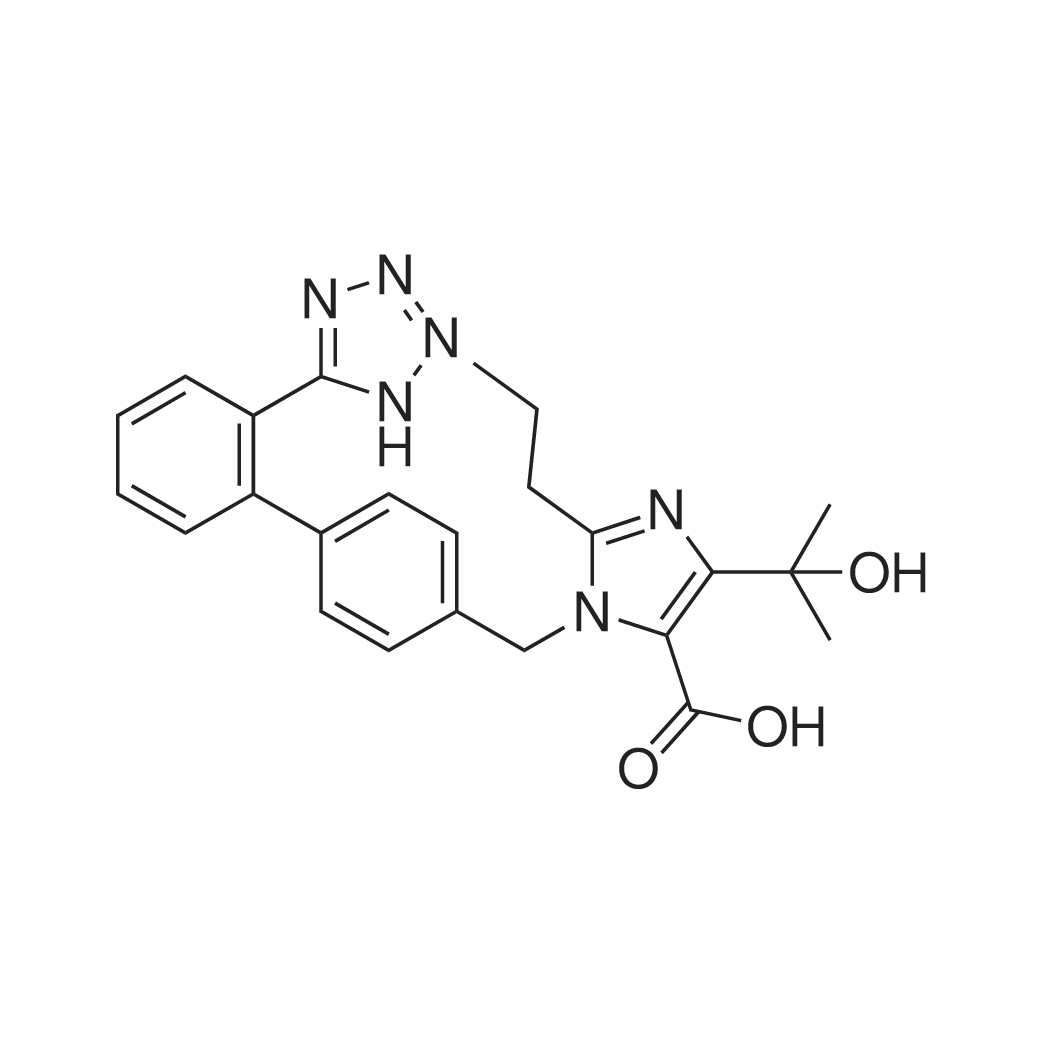
| 描述 | Angiotensin II is an important hormonal effector and end-product of the renin-angiotensin system. After binding to its endogenous receptor sites, it causes widespread vasoconstriction and a subsequent increase in blood pressure. [3]. Olmesartan (RNH-6270) is an angiotensin II receptor (AT1R) antagonist used to treat high blood pressure.[4] Olmesartan (0.7-5 mM; 24, 48 and 72 h) inhibits the growth of HeLa cells as a concentration- and time-dependent mode. [5]. A single i.v. administration of olmesartan (0.01-0.03mg/kg) produced a dose-related inhibition that reached a maximum within 30 min after dosing,followed by a gradual decline over 8h in rats.[6]. Repeated dosing of olmesartan (1 mg/kg, 2 mg/kg, p.o.) dose-dependently decreases mean arterial blood pressure (MAP) in SHR without significant influence on body weight and food intake during 10 weeks. Olmesartan (5 mg/kg/d, p.o.) and hydralazine treatments lower systolic blood pressure to the same degree in mice. Olmesartan treatment inhibits cardiac hypertrophy, evaluated by echocardiography, heart weight, cross-sectional area of cardiomyocytes, and gene expression. Olmesartan treatment reverses decreased gene expressions of ACE2 and Mas receptor of Ren-Tg mice and inhibits enhanced NADPH oxidase (Nox)4 expression and reactive oxygen species. [7]. | ||
| 作用机制 | AT1R can homodimerize and heterodimerize with other G-protein coupled receptors (GPCR), altering the receptor signaling properties. Olmesartan,biased AT1R agonists preferentially activate the β-arrestin signaling pathway.[2]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01415466 | Healthy | Phase 1 | Completed | - | Korea, Republic of ... 展开 >> Yonsei University Health System (Yuhs) Seoul, Korea, Republic of 收起 << |
| NCT00185120 | Hypertension | Phase 4 | Completed | - | United States, Alabama ... 展开 >> Mobile, Alabama, United States, 36693 United States, California Memorial Research Medical Center Long Beach, California, United States, 90806 National Research Institute Los Angeles, California, United States, 90057 Clinical Trials Research Roseville, California, United States, 95661 Apex Research Institute Santa Ana, California, United States, 92705 Orange County Research Center Tustin, California, United States, 92780 Westlake Medical Center Westlake Village, California, United States, 91361 United States, Florida Clinical Research of South Florida Coral Gables, Florida, United States, 33134 The Greater Fort Lauderdale Heart Group Research Fort lauderdale, Florida, United States, 33308 SFBC International Miami, Florida, United States, 33142 CLIRECO, Inc. Tamarac, Florida, United States, 33321 United States, Indiana Midwest Institute for Clinical Research Indianapolis, Indiana, United States, 46260 United States, Kansas Heartland Research Associates Wichita, Kansas, United States, 67207 United States, Maine Androscoggin Cardiology Research Auburn, Maine, United States, 04210 United States, North Carolina Internal Medicine Associates of Charlotte Charlotte, North Carolina, United States, 28211 Piedmont Medical Research Associates Winston-salem, North Carolina, United States, 27103 United States, Ohio The Lindner Clinical Trial Center Cincinnati, Ohio, United States, 45319 United States, Rhode Island Omega Medical Research Warwick, Rhode Island, United States, 02886 United States, Tennessee Volunteer Research Group, University of Tennessee Med. Ctr. Knoxville,, Tennessee, United States, 37920 United States, Texas Punzi Medical Center Carrollton, Texas, United States, 75006 收起 << |
| NCT01028534 | Obstructive Sleep Apnea ... 展开 >> Hypertension 收起 << | Not Applicable | Completed | - | Japan ... 展开 >> Kirigaoka Tsuda Hospital Kitakyushu, Japan Kyoto University Hospital Kyoto, Japan 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.24mL 0.45mL 0.22mL |
11.20mL 2.24mL 1.12mL |
22.40mL 4.48mL 2.24mL |
| 参考文献 |
|---|