| 生物活性 | |||
|---|---|---|---|
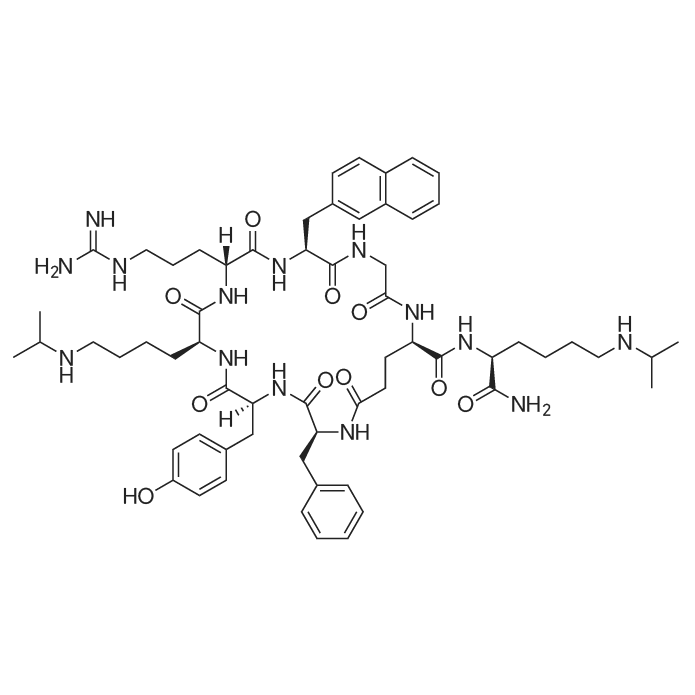
| 描述 | Through binding with CC- and CXC-chemokines, CCR and CXCR, the subsets of the chemokine receptor family can trigger an intracellular signal transduction cascade, thus mediating the recruitment of immune cells to the inflammation or infection sites and implicated in several immuno-related disease states, organogenesis, angiogenesis and tumor growth and metastasis. LY2510924 is a potent and selective CXCR4 inhibitor with IC50 value of 0.079nM for blocking SDF-1α binding to CXCR4. LY2510924 could completely inhibit SDF-1-mediated GTP binding with Kb value of 0.38nM. Selective inhibition by LY2510924 of SDF-1-induced migration of U937 cells can be achieved in a concentration-dependent manner with IC50 of 0.26nM. As prediction, the SDF-1- and CXCR4-mediated cell signaling, including p-ERK and p-AKT, can be inhibited by LY2510924 at concentration of 3.3nM and 0.33nM in HeLa cells, as well as IC50 values of 1.4 and 1.2nM, respectively, in Namalwa cells expressing high level of CXCR4. Subcutaneous injection with 3mg/kg LY2510924, BID, for 7 days, significantly inhibit the tumor growth of an NHL Namalwa xenograft model. Antitumor growth activities of LY2510924 can be also observed at dose of 2mg/kg or 5mg/kg in solid cancer xenograft models, RCC A-498 and A549 xenografts. Subcutaneous injection with 3mg/kg LY2510924 twice daily for 13 or 15 days inhibited breast cancer metastasis in the MDA-MB-231 model due to blocking migration/homing process of tumor cells to reduce the colony numbers of lung metastasis, as well as inhibiting cell proliferation to reduce the size of the colonies after tumor cell homing to the lung[1]. | ||
| 作用机制 | LY2510924 can occupy the binding pocket and possess ligand-receptor interactions with CXCR4 residues.[1] | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01439568 | Extensive Stage Small Cell Lun... 展开 >>g Carcinoma 收起 << | Phase 2 | Completed | - | United States, Colorado ... 展开 >> For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Denver, Colorado, United States, 80218 United States, Florida For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Fort Myers, Florida, United States, 33916 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Orlando, Florida, United States, 32804 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Pensacola, Florida, United States, 32503 United States, Illinois For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Chicago, Illinois, United States, 60637 United States, Maryland For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Bethesda, Maryland, United States, 20817 United States, Missouri For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Springfield, Missouri, United States, 65804 United States, Nebraska For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Omaha, Nebraska, United States, 68114 United States, Ohio For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Cincinnati, Ohio, United States, 45242 United States, South Carolina For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Greenville, South Carolina, United States, 29605 United States, Tennessee For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Chattanooga, Tennessee, United States, 37404 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Memphis, Tennessee, United States, 38120 For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Nashville, Tennessee, United States, 37203 United States, Texas For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Fort Worth, Texas, United States, 76104 United States, Virginia For additional information regarding investigative sites for this trial, contact 1-877-CTLILLY (1-877-285-4559, 1-317-615-4559) Mon - Fri from 9 AM to 5 PM Eastern Time (UTC/GMT - 5 hours, EST), or speak with your personal physician. Richmond, Virginia, United States, 23230 收起 << |
| NCT01391130 | Metastatic Clear Cell Renal Ce... 展开 >>ll Carcinoma 收起 << | Phase 2 | Terminated(Study Terminated du... 展开 >>e to Insufficient Efficacy) 收起 << | - | - |
| NCT02737072 | Solid Tumor | Phase 1 | Terminated | - | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
0.84mL 0.17mL 0.08mL |
4.20mL 0.84mL 0.42mL |
8.41mL 1.68mL 0.84mL |