| 生物活性 | |||
|---|---|---|---|
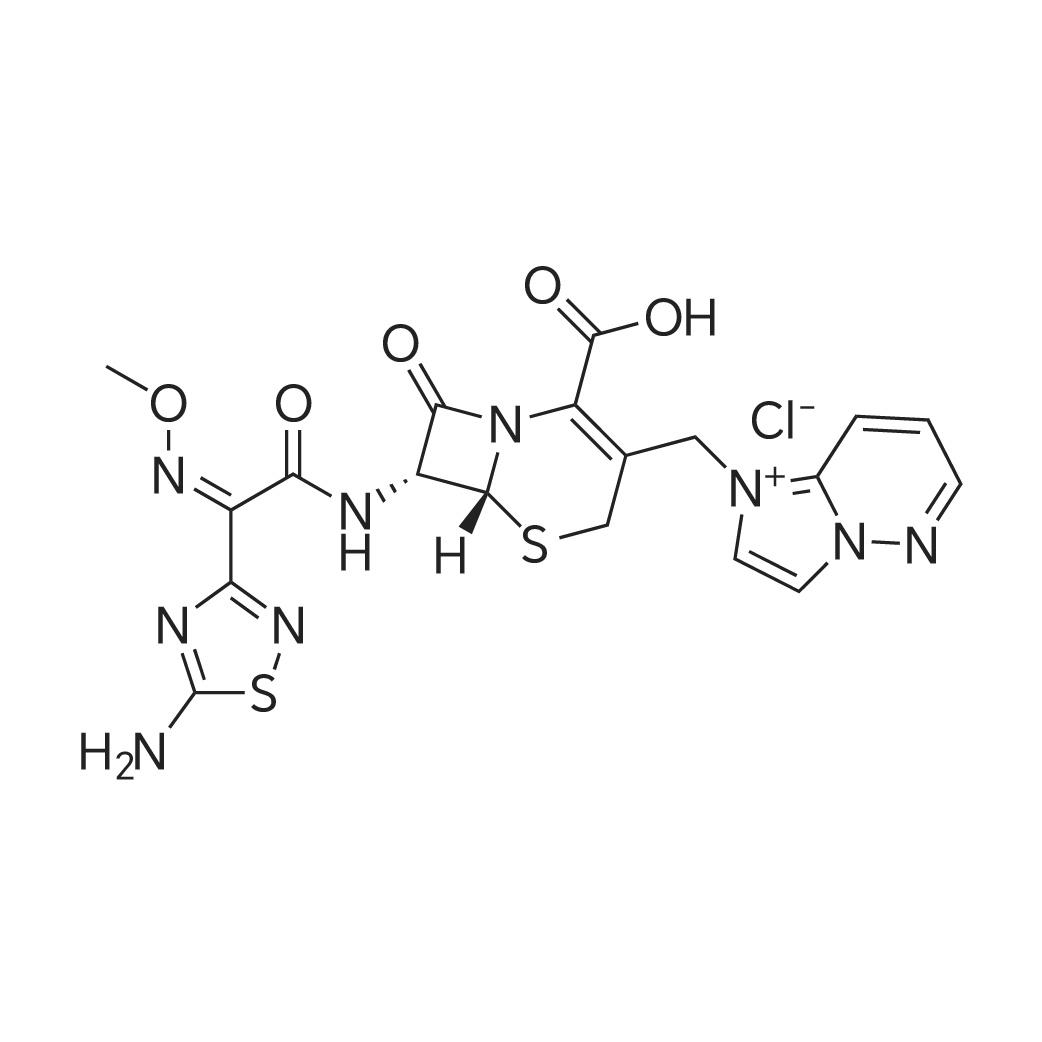
| 描述 | Cefozopran (HCl) is a parenteral cephalosporin with a broad spectrum of activity against Gram-positive and Gram-negative bacteria[3]. Cefozopran hydrochloride performs a linear kinetics in healthy volunteers. The main pharmacokinetic parameters have no significant gender differences, and there is no drug accumulated with multiple doses of injection[4]. Moreover, 20- or 40-mg/kg four times a day (0.5-h infusions), provided sufficient bactericidal effects on common bacterial populations (Escherichia coli, Streptococcus pneumoniae, Haemophilus influenzae, and Pseudomonas aeruginosa) in most typical patients[5]. Cefozopran is an effective and safe antibiotic for the treatment of febrile neutropenia in patients with hematological malignancies[6]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.81mL 0.36mL 0.18mL |
9.06mL 1.81mL 0.91mL |
18.12mL 3.62mL 1.81mL |
| 参考文献 |
|---|
|
[4]Guo WW, Shen Q, Qin YP, et al. Sichuan Da Xue Xue Bao Yi Xue Ban. 2012;43(5):711‐714 [6]Saito T, Hara M, Shinagawa K, et al. Gan To Kagaku Ryoho. 2004;31(1):61‐65 |