| 生物活性 | |||
|---|---|---|---|
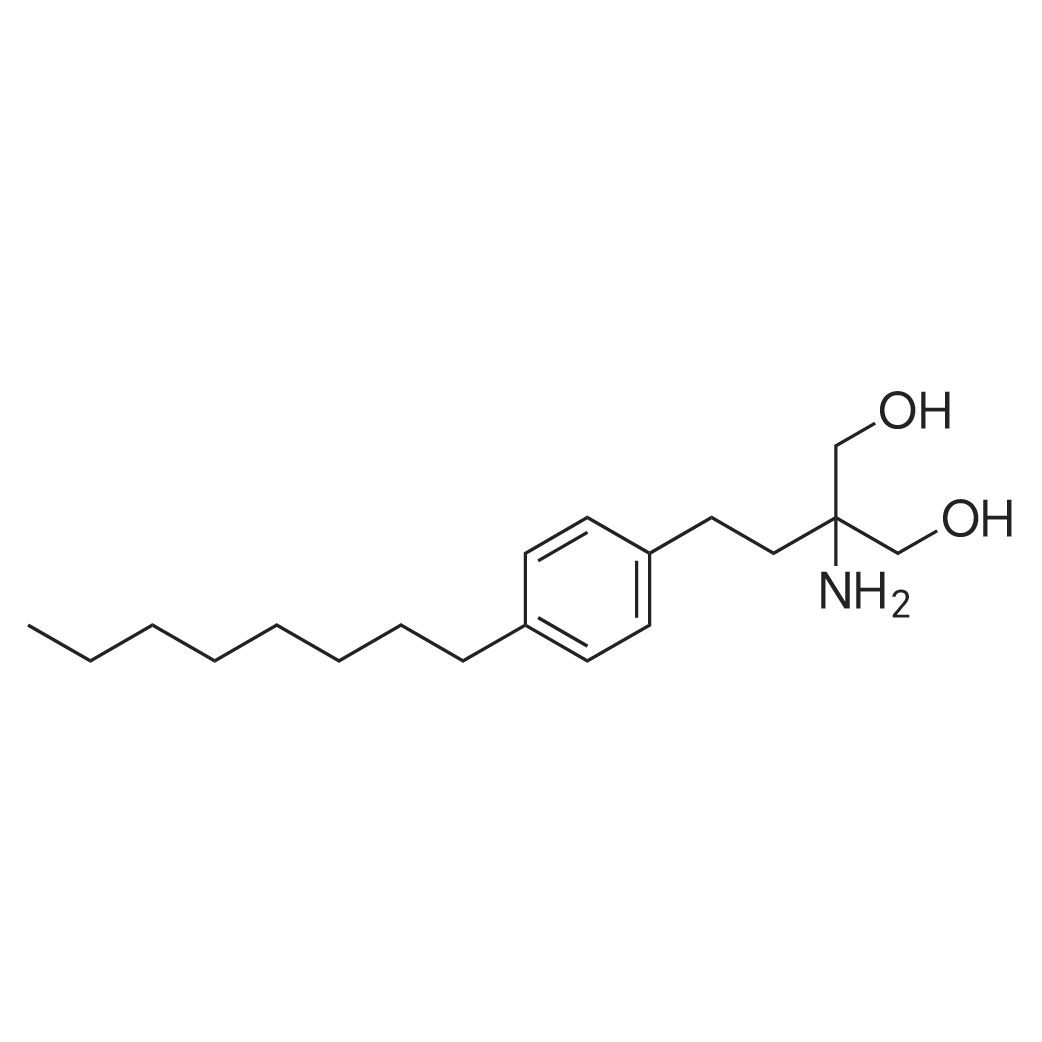
| 描述 | Fingolimod is an S1p receptor modulator and also immunosuppressant. It can be primarily phosphorylated by SPHK2 to its active form fingolimod-P. Although fingolimod-P (but not parent fingolimod) could function as an agonist at S1P1, S1P4 and S1P5 receptors with EC50 values ranging in ~0.3–0.6nM and at 10- fold higher concentrations at S1P3 receptors with EC50 values of ~3 nM[1], it caused S1P receptors to internalize from cell membranes and induce ubiquitinylation and proteasomal degradation of the receptor. That is, effects of Fingolimod are inhibitory in the longer term on S1P receptor function[2]. Fingolimod was used to treat for multiple sclerosis. Culture with FTY720 at concentration of 1μM for 22 hours could stimulate survival of the oligodendrocyte progenitors, and an activation of both MEK/ERK1/2 and PI3K/Akt pathways dependent on transforming to fingolimod-P by SphK could be observed[3]. Fingolimod is highly effective in experimental autoimmune encephalomyelitis model. Administration of fingolimod at dose of 0.03 to 1 mg/kg could significant improve the symptoms of chronic experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein in mice due to the reduction of infiltration of myelin antigen-specific Th17 and Th1 cells into the central nervous system[4]. | ||
| 作用机制 | The aminodiol polar head group of fingolimod could be phosphorylated by SPHK2 and the lipophilic tail is important for interacting with the hydrophobic binding pocket of the S1P receptors[1] | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT03535298 | Multiple Sclerosis, Relapsing-... 展开 >>Remitting 收起 << | Phase 4 | Not yet recruiting | September 2023 | United States, Ohio ... 展开 >> Cleveland Clinic Not yet recruiting Cleveland, Ohio, United States, 44195 Contact: Tammy Skaramagas, BA 216-445-6724 skaramt1@ccf.org Principal Investigator: Daniel Ontaneda, MD, MSc United Kingdom University of Nottingham Not yet recruiting Nottingham, United Kingdom, NG7 2UH Contact: Sara Wilkins +44 115 9249924 ext 66816 Sara.wilkins@nuh.nhs.uk Principal Investigator: Nikos Evangelou, MD, DPhil 收起 << |
| NCT01795872 | Multiple Sclerosis | Phase 4 | Completed | - | - |
| NCT03500328 | Multiple Sclerosis, Relapsing-... 展开 >>Remitting 收起 << | Not Applicable | Recruiting | October 31, 2022 | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.25mL 0.65mL 0.33mL |
16.26mL 3.25mL 1.63mL |
32.52mL 6.50mL 3.25mL |
| 参考文献 |
|---|