| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
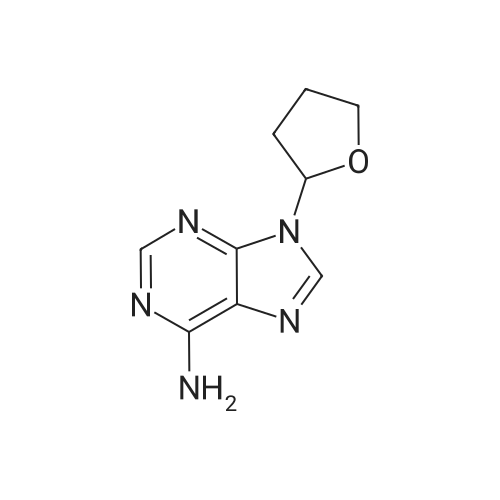
| 描述 | SQ22536 is an effective inhibitor of forskolin's effects, with IC50 values reported at 5 μM. When preincubated with various concentrations, SQ22536 also effectively suppresses PACAP-induced reporter gene activation, with an approximate IC50 value of 5 μM. It more strongly inhibits Elk activation induced by forskolin (IC50=10 μM) compared to that induced by 8-Br-cAMP (IC50=170 μM). Notably, SQ22536 exhibits significant differences in potency when inhibiting the activities of recombinant AC5 and AC6, with IC50 values of 2 μM and 360 μM, respectively. At a higher concentration of 500 μM, SQ22536 markedly inhibits neurite elongation caused by either forskolin or 8-Br-cAMP[1]. | ||
| 作用机制 | SQ 22536 is a 9-substituted adenine derivative.[1] | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
4.87mL 0.97mL 0.49mL |
24.36mL 4.87mL 2.44mL |
48.73mL 9.75mL 4.87mL |
| 参考文献 |
|---|