| 生物活性 | |||
|---|---|---|---|
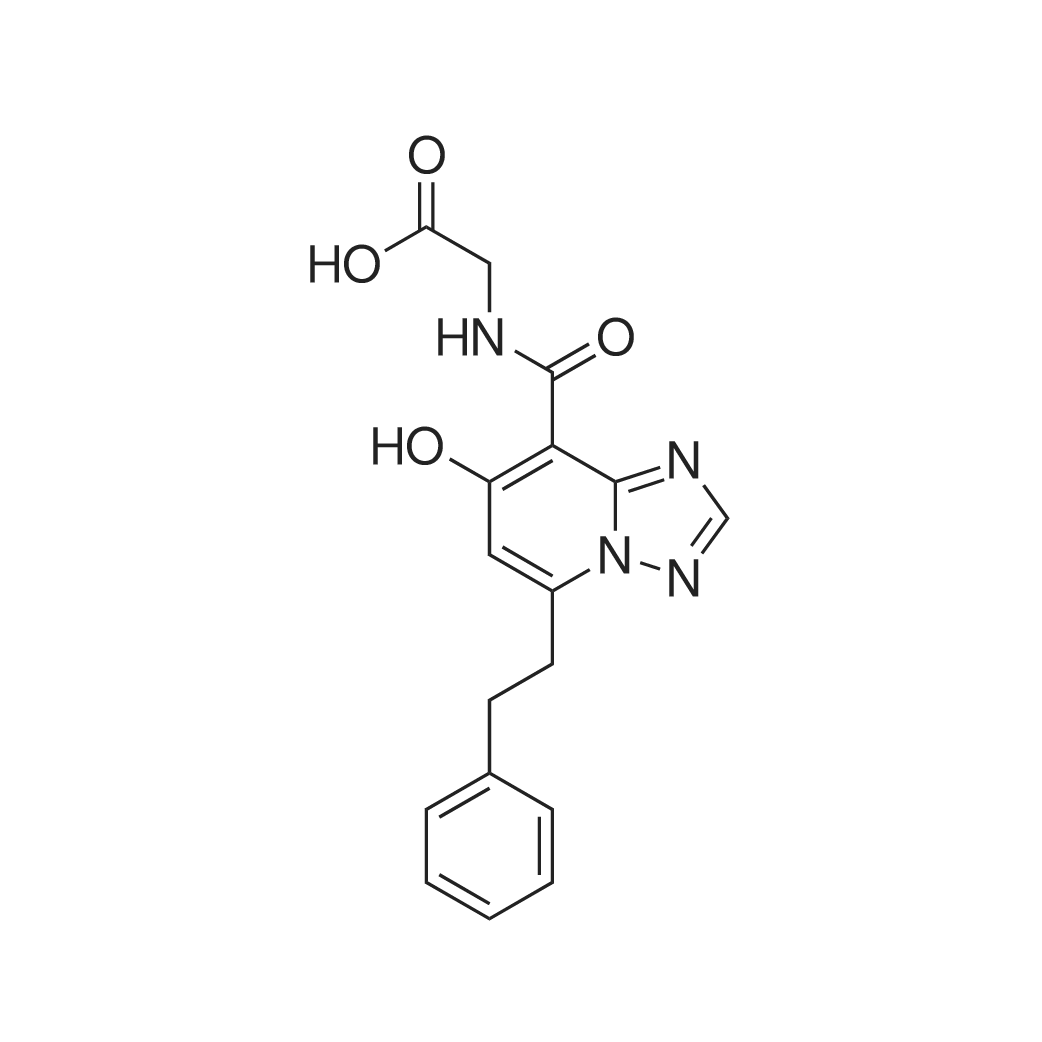
| 描述 | The hypoxia signalling pathway enables adaptation of cells to decreased oxygen availability. When oxygen becomes limiting, the central transcription factors of the pathway, hypoxia-inducible factors (HIFs), are stabilised and activated to induce the expression of hypoxia-regulated genes, thereby maintaining cellular homeostasis[1].Enarodustat (JTZ-951) is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that mimics adaptive responses to hypoxic conditions and may provide a new therapeutic approach for managing anemia in patients with chronic kidney disease (CKD)[2].Fatty acid and amino acid metabolisms were upregulated in diabetic renal tissue and downregulated by enarodustat, whereas glucose metabolism was upregulated.Whereas glycolysis and tricarboxylic acid cycle metabolites were accumulated and amino acids reduced in renal tissue of diabetic animals, these metabolic disturbances were mitigated by enarodustat. Furthermore, enarodustat increased the glutathione to glutathione disulfide ratio and relieved oxidative stress in renal tissue of diabetic animals[3].Compared with brachyury ob/ob mice that received only vehicle, BTBR ob/ob mice treated with enarodustat displayed lower body weight, reduced blood glucose levels with improved insulin sensitivity, lower total cholesterol levels, higher adiponectin levels, and less adipose tissue, as well as a tendency for lower macrophage infiltration. Enarodustat-treated mice also exhibited reduced albuminuria and amelioration of glomerular epithelial and endothelial damage[4]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.94mL 0.59mL 0.29mL |
14.69mL 2.94mL 1.47mL |
29.38mL 5.88mL 2.94mL |
| 参考文献 |
|---|