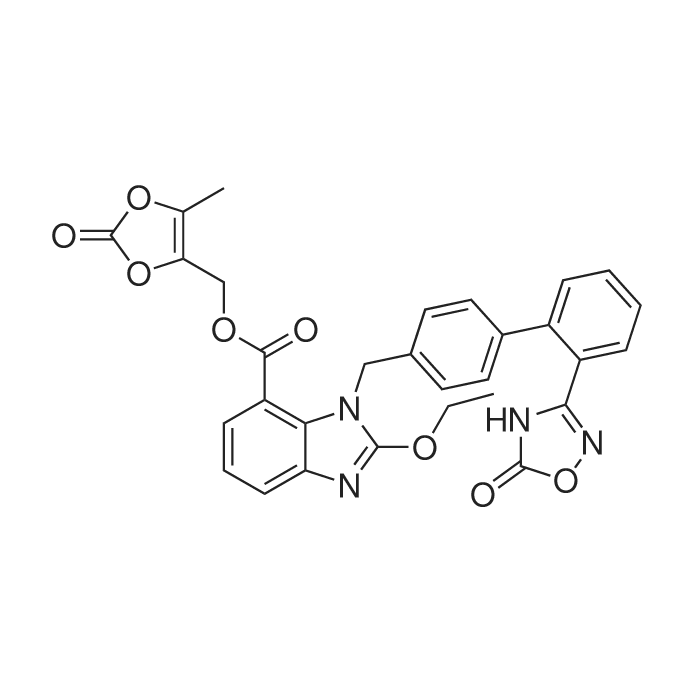
 |
同义名 : | TAK-491 |
| CAS号 : | 863031-21-4 | |
| 货号 : | A325842 | |
| 分子式 : | C30H24N4O8 | |
| 纯度 : | 95% | |
| 分子量 : | 568.53 | |
| MDL号 : | MFCD19443688 | |
| 存储条件: |
Pure form Inert atmosphere, 2-8°C In solvent -20°C:3-6个月-80°C:12个月 |
|
| 溶解度 : | - | |
| 动物实验配方: |
| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
| 描述 | Azilsartan Medoxomil, a new angiotensin II Type 1 receptor blockers with IC50 of 0.62 nM. In conscious spontaneously hypertensive rats (SHRs), oral administration of 0.1-1mg/kg azilsartan medoxomil significantly reduced blood pressure at all doses even 24h after dosing[3]. Azilsartan medoxomil, in 40 and 80 mg doses, combined with 5 mg of the calcium channel blocker amlodipine was well tolerated and led to meaningful additional BP(blood pressure) reductions compared with placebo plus amlodipine[4]. Further, in aortic endothelial cells, azilsartan inhibited cell proliferation at concentrations as low as 1 μmol/l. Antiproliferative effects of azilsartan were also observed in cells lacking AT1(angiotensin II type 1) receptors[5]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT01715584 | Hypertension | Phase 4 | Recruiting | December 31, 2019 | Canada, Ontario ... 展开 >> London Health Sciences Centre - Victoria Campus Recruiting London, Ontario, Canada, N6A 5W9 Contact: Craig J Railton, MD, PhD 519 685 8500 ext 58525 Craig.Railton@lhsc.on.ca Principal Investigator: Craig J Railton, MD, PhD Sub-Investigator: Jonathan Fairbairn, BSc Sub-Investigator: George Nicoloau, MD Sub-Investigator: Robert Gros, PhD Sub-Investigator: Jason Franklin, MD Sub-Investigator: John Yoo, MD Sub-Investigator: Kevin Fung, MD Sub-Investigator: Anthony Nichols, MD Sub-Investigator: Danielle McNeil, MD 收起 << |
| NCT02235909 | Hypertension | Phase 3 | Recruiting | August 2020 | - |
| NCT00762736 | Diabetes Mellitus | Phase 2 | Completed | - | - |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.76mL 0.35mL 0.18mL |
8.79mL 1.76mL 0.88mL |
17.59mL 3.52mL 1.76mL |
| 参考文献 |
|---|

