| 生物活性 | |||
|---|---|---|---|
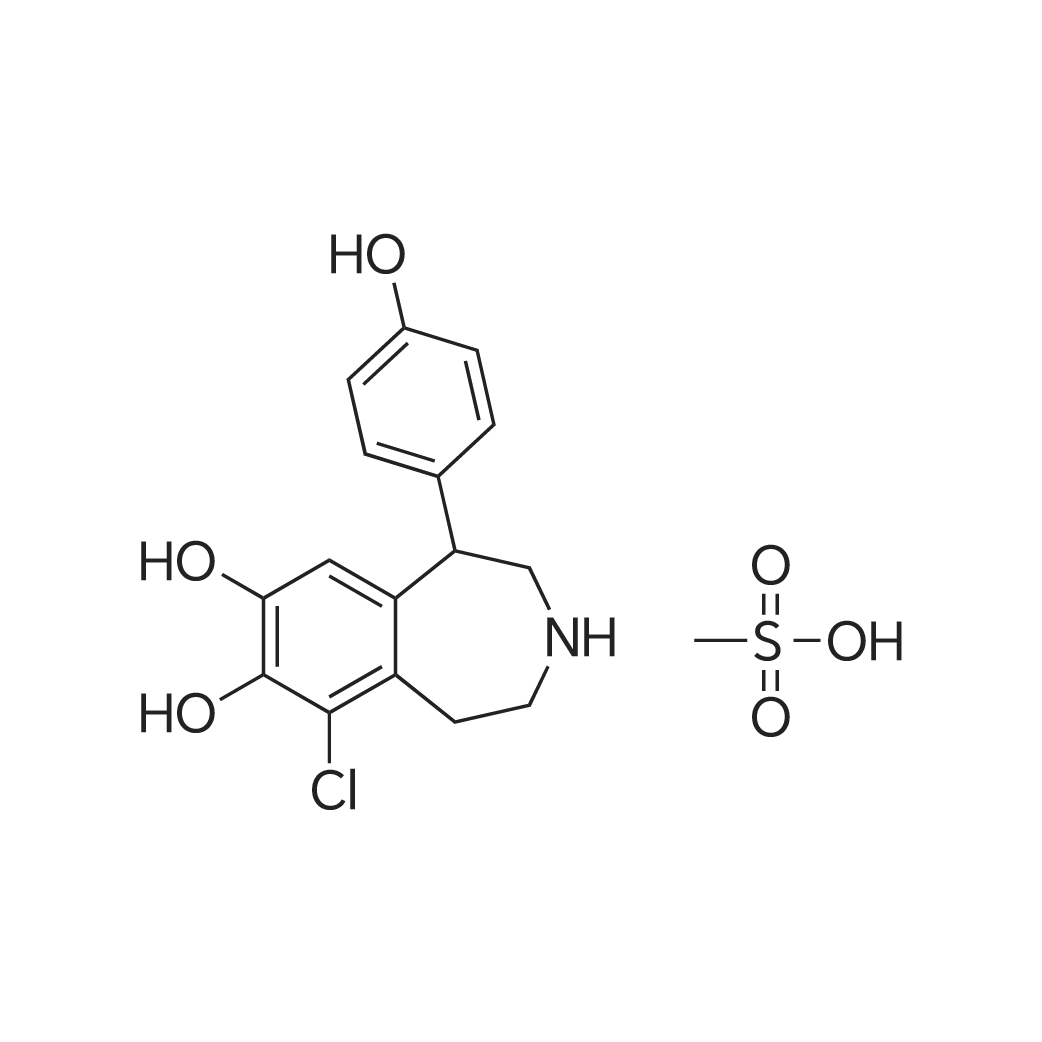
| 描述 | Fenoldopam mesylate is a selective agonist of DA-1 receptors. It is currently used for the in-hospital treatment of severe hypertension. Fenoldopam mesylate was found to be effective in reducing the onset of postoperative AKI (Acute Kidney Injury), when used before the development of the kidney damage[3]. At higher doses, fenoldopam lowers blood pressure but still maintains renal perfusion. In addition to its renal vasodilator activity, fenoldopam is natriuretic, possibly resulting from a direct effect of DA1 receptors on the proximal convoluted tubule. In animals with spontaneous or drug-induced renal failure, fenoldopam improves renal function[4]. In hemodynamically stable cardiac surgery patients with preserved renal function, an infusion of 0.1 mg/kg/min of fenoldopam mesylate has no influence on systemic hemodynamics while increasing renal blood flow[5]. Fenoldopam CRI at 0.1 µg/kg/min did not have a clinically relevant effect on kidney function parameters in dogs with severe heatstroke-associated AKI[6]. The selective dopamine-1 agonist fenoldopam mesylate does not prevent further renal function deterioration after contrast administration in patients with chronic renal insufficiency[7]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00557219 | Acute Renal Failure | Phase 3 | Terminated(Main cooperator fin... 展开 >>ished cooperation) 收起 << | - | Poland ... 展开 >> Department of Cardiac Anesthesiology, Medical University of Gdańsk Gdańsk, Poland, 80-211 收起 << |
| NCT00122018 | Kidney Failure, Acute ... 展开 >> Kidney Failure, Chronic Cardiac Surgical Procedures 收起 << | Phase 2 | Completed | - | - |
| NCT01690832 | Coronary Artery Disease | Phase 4 | Unknown | December 2014 | Italy ... 展开 >> Sapienza University Rome, Lazio, Italy, 00166 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.49mL 0.50mL 0.25mL |
12.44mL 2.49mL 1.24mL |
24.88mL 4.98mL 2.49mL |
| 参考文献 |
|---|